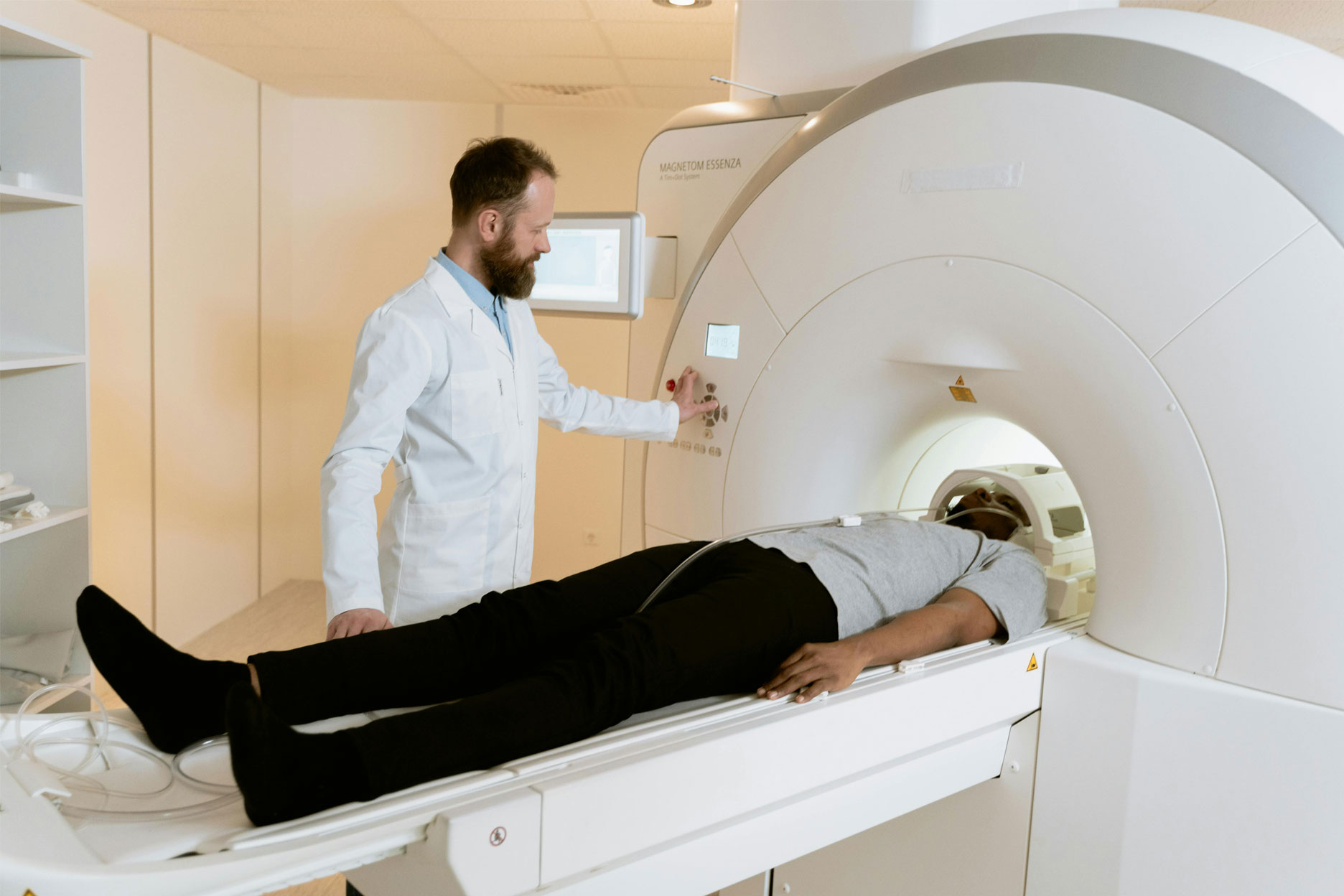
We are regulatory consultants with a wealth of expertise, having vast consultancy experience in addition to our Managing Director’s origins as a medical device manufacturer. We provide expert medical device regulatory guidance to help launch products and access new markets.
KD&A specialise in providing a range of medical device regulatory services to manufacturers, sponsors, and distributors of medical devices, including IVD devices.
- Medical device regulatory strategy report and roadmap to market.
- ISO 13485 QMS development, maintenance, internal auditing and support including regulator/ Notified Body audits.
- Global medical device registrations; EU CE Marking, Australia TGA Conformity Assessment and Australian Register of Therapeutic Goods (ARTG) inclusions, US FDA, Health Canada, UK UKCA, Singapore HSA, etc.
- Development of device specific Technical File documentation for medical devices and IVD devices.
- TGA sponsor services.
- Post market surveillance including reporting to the TGA.